Abstract
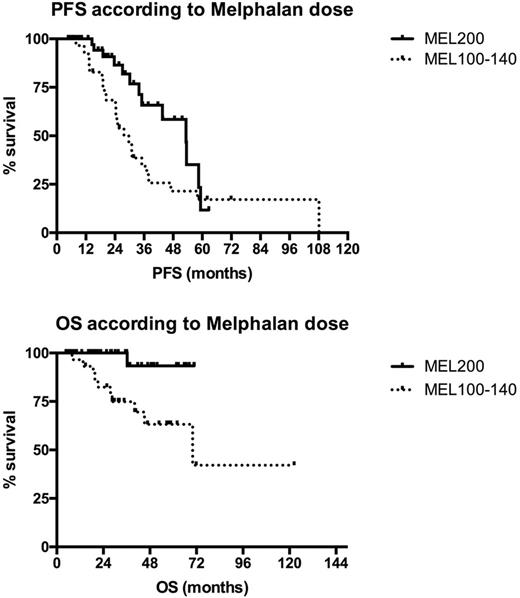
Introduction: The age limit of 65 for performing Autologous stem cell transplantation (ASCT) in elderly patients with Multiple Myeloma (MM) is currently debated, since efficacy and safety have been recently improved but no conclusive data are still available. Over the last years the policy of our Center was to treat fit patients up to the age of 75 with the same program adopted for younger patients, i.e. bortezomib-based induction followed by ASCT. Herein we report the outcome of the series of consecutive patients aged >65 undergoing first-line ASCT at our center together with an analysis of potential prognostic parameters.Patients and Methods: From January 2009 to December 2016 86 of 270 (31,8%) newly diagnosed symptomatic MM patients aged 65-75 years (M/F: 46/40 , median age 68, 35% aged ≥ 70) received bortezomib-based induction (VTD 91%, VD 7%, VCD 2%) followed by ASCT as first-line treatment. Standard conditioning was melphalan 200 mg/sqm (MEL200) and patients not achieving CR after ASCT were to receive a second ASCT 3-6 months after the first if grade IV non hematological toxicities were not observed with first transplant. According to physician judgement based on age, comorbidities and fitness, MEL dose could be reduced to 140 or to 100mg/sqm. Overall 38% of patients received a single and 62% a double transplant and MEL200 was given to 63%, MEL140 to 9% and MEL100 to 28% of patients, respectively. ECOG PS at diagnosis was ≥2 in 21% of patients. The impact of age, ECOG PS and MEL dosage on progression-free (PFS) and overall survival (OS) was analyzed. Results: Complete remission (CR) after the first ASCT was 34% and very good partial response (VGPR) was 39%. Rate of grade 4 non-hematologic toxicity was 16% (5% mucositis, 10% infections; one Idiopathic Pneumonia Syndrome 60 days after ASCT was also observed). Median hospital stay was similar between patients aged 65-69 years and ≥ 70 years (18 vs 18 days). Transplant related mortality (TRM) was 0%; death rate was 13% and was due to progressive disease in 91% of cases (the latter 9% concerns a cardiac arrest secondary to aspiration pneumonia in a patient in VGPR 3 months after first ASCT). After a median follow-up of 2.5 years, 2-year PFS was 75% and OS 91%. No significant survival difference was seen between patients aged 65-69 and ≥ 70 years (2-year PFS 75% vs 70% and OS 92% vs 91%, respectively). PS 0-1 vs ≥ 2 at diagnosis significantly impacted on OS (2-year PFS 74% vs 60%, p=0.65; OS 97% vs 69%, p 0.01, respectively). A significantly better outcome was seen in patients receiving MEL200 conditioning, compared to lower MEL doses (2-year PFS 86% vs 57%, p 0.036; 2-year OS 100% vs 82%, p 0.005; Figure 1). In the subgroup of 51 patients receiving VTD and MEL200 median OS was not reached. Of note, 44% of patients in the PS ≥ 2 subgroup received MEL200, suggesting that impaired long term survival is only partially influenced by a less intensive treatment. Conclusion: According to our single center experience a significant proportion of newly diagnosed MM patients aged up to 75 years can successfully receive a bortezomib-based (VTD) induction regimen followed by ASCT. Efficacy is highest with MEL200 as conditioning before ASCT and survival Is better in patients with PS <2. Objective methods are warranted to assess transplant eligibility and the optimal dosage of the conditioning regimen in the single patient in order to optimize the use and the efficacy of ASCT in elderly MM.
Figure 1: outcome according to MEL dosage
Rossi: Celgene: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Teva: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal